Lesser Sunda Islands Part 1 – Flores & Sumba
Introduction
The Komodo Dragon (Varanus komodoensis) looms in the public conscious. Dwarfing all other extant lizards (not including snakes), capable of subduing oxen and notoriously even taking human lives. A handful of these behemoth saurians dotted in remote refugias are all that remains of the once distinctive community of the Lesser Sundas, an archipelago located east of Java and comprising Bali, Lombok, Sumbawa, Flores, Sumba, Timor, and a host of smaller isles. Biogeographically these islands are highly significant, they are part of an intermediary zone between Sahul and Asia, separated from both by narrow - but deep - straits. Precious few species penetrated this barrier (5), resulting in a poor vertebrate diversity – comprised of adept colonizers - with an impressive longevity throughout the Pleistocene (10, 28). These arrivals came from Sundaland to the West, Sahul to the East and South and Sulawesi to the North, creating hybrid faunal guilds. Disregarding orthodox proportions altogether, the denizens of the Sundas consisted of tiny giants and critters which attained awesome dimensions. Man-sized storks towered over dwarfish stegodonts – Peculiar relatives of the Elephant. Our own minutest of cousins, fearfully foraging the bush – paying heed not to attract prowling dragons. Of the original fauna of the Lesser Sundas, no animal exceeding a few kilograms – barring the Komodo Dragon – survive to this day. What calamitous event transpired, which could deplete the islands so dramatically? To answer this question, one must look to the fossil records of the individual islands, examining the evidence of megafauna, extinction, climate change and human arrival and influence. After which an aggregate picture will form, consolidating evidence from each part of the archipelago. This exploration will be divided into two instalments, the first of which focuses on Flores – the most fossiliferous of the Lesser Sunda Islands, as well as its neighbour of Sumba. The second part will be devoted primarily to Timor, though the miscellaneous islands will also be briefly discussed. Bali was connected to nearby Java during times of low sea level, for this reason it is biogeographically different and will be omitted.
Starting then with Flores and Sumba. Flores sits around the middle of the archipelago and Sumba is located immediately to the south of it. During the Pleistocene Flores would have been connected directly to the adjacent Komodo islands (30). It has been suggested that a land bridge between Flores and Sumba existed sometime in the Pleistocene, owing to a very similar faunal guild, however current understanding of stratigraphy does not support this (24, 28).
Flores constitutes the most exhaustively studied island of the Lesser Sundas; indeed, it is from here we derive the bulk of our knowledge. The sites of Mata Menge and especially Liang Bua have yielded a treasure trove of fossils through a well-defined stratigraphy enabling a tracking of taxa through time – allowing corroboration with extinction hypotheses. But before diving into those hypotheses, it is important to gain an overview of the extinct fauna present on Flores and Sumba.
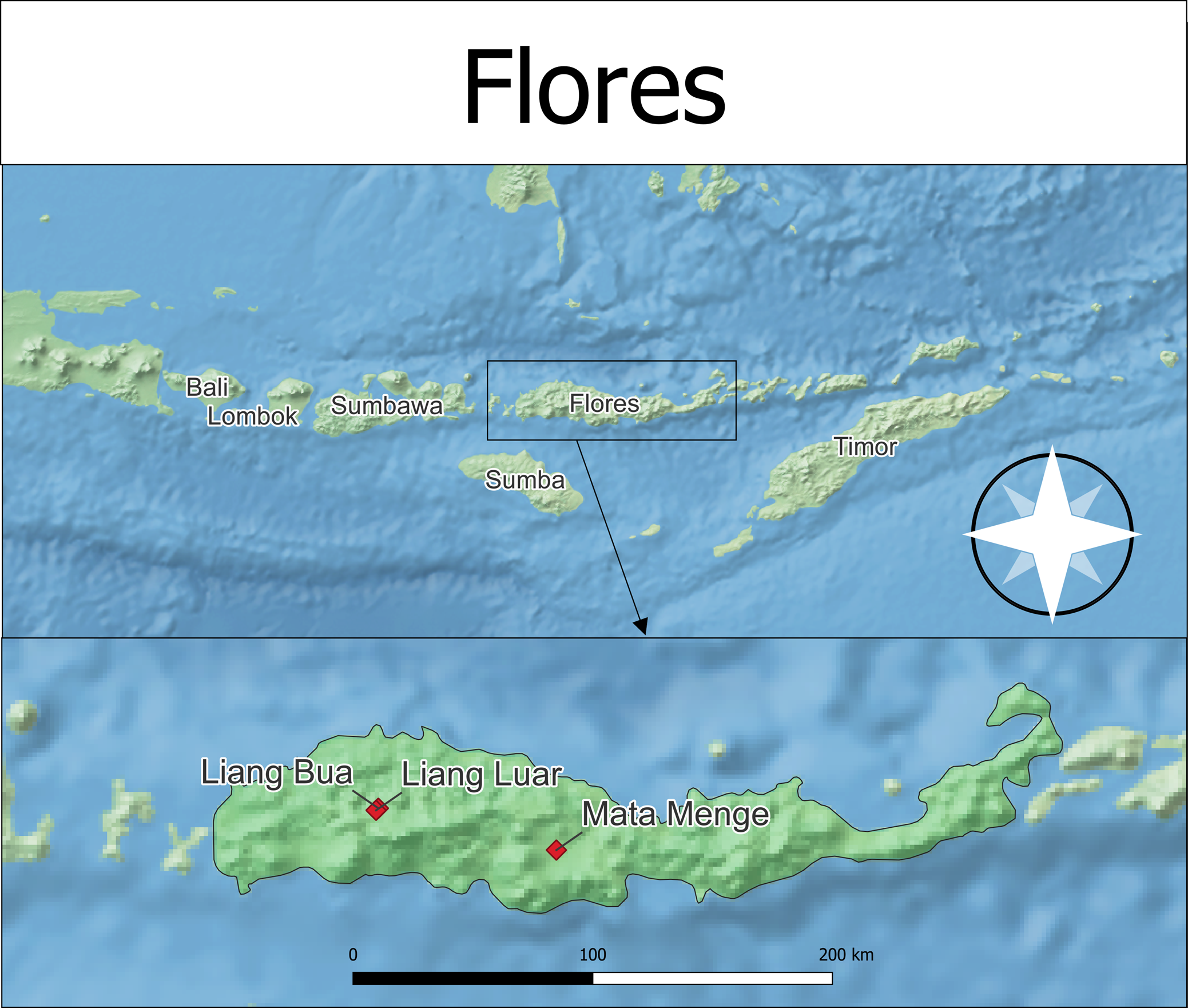
Figure 1. Map of Flores in the Lesser Sundas, along with sites mentioned in the text.
Terms of use: Own work
The Faunal Overview
The preeminent grazers in Flores and Sumba were the stegodonts, a family of proboscideans known from Asia during the Pleistocene and especially prolific as dwarf insular species (30). Two species are known from Flores. Stegodon sondaari dates to the Early Pleistocene and attained a mass of about 490kg (30, 31). S. sondaari appears to have been displaced by the comparatively colossal (∼1700kg) Stegodon floresis during the Middle Pleistocene (30, 31, 34), though by the Late Pleistocene the species had shrunk to just 570kg (31). On Sumba a few fragments of a species of Stegodon have been tentatively designated as Stegodon sumbaensis which resembles S. sondaari (24, 28) but attaining even more miniscule dimensions – perhaps weighing as little as 250kg (31). S. sumbaensis is known from deposits extending into the Late Pleistocene (28).
Likely preying upon these dwarf stegodonts was the Komodo dragon (Varanus komodoensis) which undoubtedly filled the niche of apex predator on Flores (30), and still does so on the fringes of the island (6). Remains confidently assigned to V. komodoensis have also been discovered on Sumba (28). A much smaller extinct species of monitor lizard is also known Flores, Varanus hoojeri - which lacks a common name but we shall refer to as the Flores monitor (1, 30). The Flores monitor differs heavily from the komodo dragon in niche, showing morphological affinities to the Nile monitor (Varanus niloticus) and Gray’s monitor (Varanus olivaceus) (1) - both of which are omnivorous. It has been suggested that like Gray’s monitor, the Flores monitor may have subsisted primarily on fruit (1, 30). Fossil remains of a varanid similar in morphology to (and likely conspecific with) the Flores monitor have also been found on Sumba (24)
Figure 2. The Komodo Dragon (Varanus komodoensis) is the only surviving megafauna species on Flores and its apex predator.
Terms of use: This image is licensed under a Creative Commons Attribution 2.0 Generic. It is attributed to Mark Dumont. The image is unedited and can be found here.
Another sizeable reptile from Flores is the giant tortoise Collossochelys azizi (30). C. azizi is restricted to a single stratum in the Early Pleistocene (21) and be considered a nomen nudum (21) meaning it was described with insufficient detail. The species may in fact be an unnamed species of Megalochelys. A single giant tortoise bone was reported from Sumba but has since been lost – its taxonomic affinities and stratigraphy are unknown (24). Rounding out the large reptile roster is an unknown crocodile. Fossil remains are scant and fragmentary, thus a taxonomic assignment is impossible. Their presence in inland freshwater bodies might indicate an extinct native species (4).
Looking at Avifauna, birds at Liang Bua have recently been surveyed, unearthing several extinct species (11). Largest of all, was Leptoptilos robustus (9, 11, 15) a relative of the Marabou stork (L. crumenifer) and adjucants (L. javanicus & dubius)(9, 11, 15). Standing 1.8m tall, the species is thought to have subsisted on small animals and carrion (9). Remains of a vulture of the genus Trigonoceps have also been uncovered at Liang Bua – currently this genus is only represented by the white-headed vulture (T. occipitalis) from sub-Saharan Africa (11). Remains indicate both the presence of a large barn owl (Tyto sp.) (11, 30) and a possibly flightless species of crake (Porzana sp.) (11). Two extinct passerine taxa were discovered: a grassbird (Megalurus/Poodytes), and a starling, likely of the genus Acridotheres (14). Whether these are endemic species or subspecies is unclear (14). No bird fossils have been described from Sumba.
Figure 3. The Striated Grassbird (Megalurus palustris) is the only species of the genus Megalurus and inhabits much of South Asia, including Java. It is a candidate for the extinct remains described Meijer et al 2017.
Terms of use: This image is licensed under a Creative Commons Attribution 2.0 Generic. It is attributed to Imran Shah. The image is unedited and can be found here.
Findings from Liang Bua suggest that murids (mice & rats) were the most abundant vertebrates on Flores, comprising about 80% of all fossils finds from the site (33), though they are subject to accumulation biases from predation (33). Several giant rats were present on Flores. One species still survives to this day, the Flores giant rat (Papagomys armandvillei), which also shares an extinct relative of the same genus Papagomys theodorverhoeveni (7, 17), these species were ground-dwelling and tree-dwelling respectively (17). A third species in the genus has also supposedly been found but remains undescribed (33). P. armandvillei is the largest of the Flores murids, reaching approximately 2.5kg (33). Additionally, are the extinct species Spelaeomys florensis (7, 17) and Hooijeromys nusatenggara. This latter species is described only from the Middle Pleistocene (7, 17), but similar remains have recently been found in the Late Pleistocene (33). There are also the medium-sized Floresian rat species Paulamys naso – a lazarus taxon – and Komodomys rintjanus now found only on Komodo (7, 16, 17). Two extinct species of giant murid have recently been described from Sumba: Milimonggamys juliae and Raksasamys tikusbesar, which both appear closely related to the giant murids of Flores, the latter especially grouping with Papagomys (28). An undescribed giant shrew rat is also represented in Flores, though its remains are relatively rare (19). Two extinct shrews are also known from Liang Bua, though neither have received a formal description yet due to inadequate material (18). Two bat species found at Liang Bua are also no longer present on Flores (20)
Flores has a rich history of hominids, with the earliest known evidence of tool use dating to about a million years ago (3). The identity of these original colonizers remains a contentious issue, one possibility is Homo erectus (29) though other proponents favour a more primitive species akin to Homo habilis (29). Whichever hominid first reached Flores, they subsequently evolved into a dwarf species known as Flores man (Homo floresiensis) (2, 30). The earliest remains of H. floresiensis are known from 700kya (4). Flores man appears endemic to the island and no other hominids are known from the Lesser Sundas until the arrival of Homo sapiens.
Figure 4. Late Pleistocene Floresian landscape featuring, from left to right, a group of Flores Hobbits (Homo floresiensis), the Flores Monitor (Varanus hoojeri)., Flores giant rats (Papagomys armandvillei), giant storks (Leptoptilos robustus), Floresian vulture (Trigonoceps sp.), Floresian dwarf stegodons (Stegodon floresis)
Terms of use: Artwork by Hodari Nundu and Commissioned by The Extinctions
Extinction
The first notable extinctions are the demise of the giant tortoise Collossochelys azizi and Stegodon sondaari, which has been associated with the appearance of hominid tools approximately 0.9 mya (3). Many giant tortoise extinctions have been suggested to be due to hominid or, at the very least, human exploitation during the Pleistocene and Holocene (21). However, the discovery of hominid tools which predate this extinction by about a hundred thousand years (3) does throw this narrative into question. Likewise, in Java the arrival of Homo erectus appears to predate the extinction of the endemic giant tortoise (21). Of course this does not preclude a hominid cause entirely, however a period of co-existence of a hundred thousand years would certainly indicate a different intervention. One possible hominid based explanation would be the emergence of Homo florensiensis from the original hominid coloniser, which likely would have existed in higher concentrations and may have applied a higher hunting pressure on tortoises. Though the diet of H. floresiensis is still unclear, the structure of the teeth and jaw, as well as wear marks are consistent with carnivory (Cook et al 2021).
Alternatively, a series of volcanic eruptions taking place around 900kya may have been the root cause of the extinction of both the large herbivores (3). Both large proboscideans and tortoises have long lifespans and slow rates of reproduction, it is possible that their populations were dwindled down sufficiently by the catastrophes, after which hunting pressure proved too high. This may be especially true since both Komodo dragons (6) and probably Homo floresiensis were capable of subsisting on a wide range of prey and may have rebounded more quickly following the eruptions. Perhaps, Stegodon sondaari underwent a genetic bottleneck and could be displaced by an incoming colonisation of Stegodon that would give rise to Stegodon florensis.
Of course, another possibility is that Collossochelys azizi did not go extinct at this time at all. As noted earlier, the species is only known from a single fossil on Flores, and care should always be taken not to extrapolate extinction dates from the last appearance of a fossil, especially when remains are scant. Might the giant tortoise have succumbed along with the other large animals? Given the wealth of fossils we have from the Late Pleistocene, it is unlikely, but possible. Perhaps the influx of Stegodon floresis was sufficient cause to displace Stegodon sondaari and Collossochelys went extinct at a different time for an altogether different reason, be it overhunting, environmental changes, disease, or some confluence of these factors. With present evidence, it is impossible to say.
However, the disappearance of Stegodon sondaari and Collossochelys azizi were merely the prelude to the much more significant collapse which occurred during the Late Pleistocene. Key to understanding this event, and the palaeontology of Flores more broadly, is the primary fossil site – Liang Bua – From which we derive knowledge of all Late Pleistocene species on the island. Liang Bua is a cave located in central Flores with fossil-bearing stratigraphy stretching from the modern day to about 190kya, spanning 8 discrete ‘units’ of differing time intervals. Layers are primarily comprised of fossiliferous silt though punctuated with fossil-lacking tephra – layers formed during volcanic eruptions (27). The individual layers have fortunately been thoroughly dated, allowing some clarity to the timeline of events at the site (27). The megafauna extinctions of Flores had previously been erroneously dated to about 17kya or 13-11kya (25), however in 2016 this paradigm was shifted by Sutikna et al who redated the stratigraphy and remains at Liang Bua (25, 27). The reanalysis of the deposits as well as direct dating of Homo floresiensis have placed its fossils at Liang Bua in a temporal range of 100-60kya (Unit 1b), although stone artefacts associated with the species extend from the earliest deposits until about 50kya (Units 1a-2) (25, 27). This determination of stone artefacts is based on the rock type silicified tuff being largely disused after 50kya.
Figure 5. Photograph of the interior of Liang Bua, the most important fossil site in the Lesser Sundas.
Terms of use: This image is licensed under a Creative Commons Attribution 2.0 Generic. It is attributed to Rosino. The image is unedited and can be found here.
It is suggested that Stegodon florensis too went extinct around this time, although in this case the evidence is a little murkier. The vast majority of material assigned to this species are found in the same deposits as H. floresiensis and its artefacts, often quite abundantly - accounting for up to 6.8% of fossils. But a very few fossils have been found in later layers, comprising in one layer 0.02% of animal remains (27). One bone was even found in a late Holocene stratum! At first glance this may suggest a late survival of Stegodon into recent times, however caution should be taken in this interpretation as erosion and disturbance of the site could also account for these stray occurrences (27) and did Stegodon indeed survive into the Late Holocene of Flores, it should be attested in historical records or oral tradition. It is not. Sutikna et al 2018 proposes that Stegodon likely met its demise at the same time as Homo floresiensis around 60-50kya, but further evidence will be needed to resolve the extinction date.
The disappearance of Leptoptilos robustus and Trigonops sp. coincide with the disappearance of Homo floresiensis and the tentative extinction of Stegodon floresis (11, 27) and likely was caused by the disappearance of large carrion, which in all probably made up the majority of the diet of both species (9, 11, 12, 15). Given that Stegodon is the only known large herbivore from Late Pleistocene Flores, it is not unreasonable to suggest that the loss of this keystone species caused the demise of the two large birds.
What might have caused the extinction of all large animals on Flores, barring the Komodo dragon? There are effectively three hypotheses to contend with: volcanic eruption, climate change, and the arrival of modern humans (Homo sapiens).
At Liang Bua there is strong evidence of volcanic eruptions occurring around the time of the extinction of the Flores megafauna. Volcanic eruptions near Liang Bua have put down distinct layers of tephra, enabling a clear overview of the volcanic activity. One particularly thick layer of tephra (Unit 3) separates the last layer with megafauna clearly present (Unit 2) and a subsequent layer lacking evidence of Homo floresiensis, Leptoptilos robustus, Trigonops sp., and where Stegodon remains are incredibly scarce (Unit 4) (27). Volcanic eruption on an island then is seemingly a plausible explanation, however this hypothesis is not without major flaws. The volcanic eruption coinciding with the extinctions does appear to have deposited more material at Liang Bua than any other event, this could certainly suggest a dramatic period of volcanic activity but may also be a product of proximity to the volcano in question or differences in sedimentation rate (e.g., from an especially rainy period). It should also be noted that volcanic eruptions aren’t new to Flores by any means, other layers of tephra have been found at Liang Bua and at other sites on the island (3, 27) Most glaringly, it would be an explanation exclusive to Flores and thereby ignores the context of regional megafauna extinctions. If we accept that Flores’ megafauna was wiped out in a volcanic eruption it would require a separate explanation for the loss of Sumba’s megafauna despite the two faunas being very similar. As we shall see in part 2, the other islands of the Lesser Sundas also suffered megafauna extinctions, thus a volcanic explanation is not parsimonious. Though a contributory role of a volcanic eruption can’t be excluded, it is very unlikely to be the causal trigger of the extinctions. Could this causal trigger have been changes in the climate of Flores?
Unfortunately, our focus on climate change on Flores has been concentrated in the last 50,000 years to explain the previous erroneously dated megafauna extinction, and even these climatic studies on the island are few and far between. Furthermore, studies on sedimentation rate at Liang Bua using the incorrect dating of layers are now obsolete (27, 32). A now largely outdated study by Westaway et al 2009, did however demonstrate that there was climatic variability in rainfall at Liang Bua throughout the Late Pleistocene, but the periods of this rainfall now remain uncertain.
A nearby cave named Liang Luar has however yielded information on local climatic conditions by analysing speleothems - that is the layering of mineral deposits in caves – looking at Oxygen-18 and Carbon-13 isotopic data. The former isotope allows a tracking of temperature conditions and the latter the vegetation type (22, 23). At Liang Luar the most marked difference occurred during the Pleistocene-Holocene transition (22, 23) with an increase in humidity and a major environmental change around 68kya towards a drier more open climate (22, 23), though with a subsequent movement back to more closed forested conditions around (22, 23). This event does seem to predate the extinction of the megafauna substantially and more studies are needed to confirm that this shift in conditions is not simply reflecting a local phenomenon. Veatch et al 2019 suggests that changes in habitat type away from open landscapes towards closed forest may have caused the disappearance of the megafauna at Liang Bua, not through extinction at that time but perhaps simply because of migration, however this is quite an extraordinary claim. Another way to evaluate a climate change extinction hypothesis would be through the impacts that we might expect. One prediction that can be tested is that a large shift in the species makeup of Liang Bua would follow from the kind of radical climatic shift needed to explain the megafauna extinction of Flores, and this was carried out by Sutikna et al 2018. The findings, which were based on 284,689 distinct classified remains suggested very little change in the species composition of Liang Bua during the period where megafauna disappears (Units 2-4), suggesting an extinction selective of large animals. Indeed, the species composition does change radically at the Pleistocene/Holocene transition where a global change in climate is well attested, but by this time the megafauna is long gone (27). Ultimately, climatic studies are sorely lacking in the light of a new stratigraphy at Liang Bua so examining their impact is exceedingly difficult, however as of 2023 there is little substantial evidence to suggest a dramatic climatic shift on the island around the time of the extinction of the Flores megafauna.
We finally turn our attention to the last hypothesis: the arrival of Homo sapiens. Unfortunately, Liang Bua lacks any remains undoubtedly of human origin prior to the Holocene. Nevertheless, Sutikna et al 2016 suggests the change in rock from primarily silicified tuff to primarily chert could account from a displacement in the hominid species from Homo floresiensis to Homo sapiens, furthermore these later stone artefacts much more commonly show evidence of being exposed to fire (26). This transition does seemingly occur at the same time as the extinction of the Flores megafauna (Silicified tuff in Unit 1-2 and Chert in units 4 and younger), suggesting a link. An alternative explanation could be a shift in Homo floresiensis technology. This appears unlikely though when considering the regional context. As we shall see in part 2 of this series, modern humans were present on Timor by at least 42kya despite lying to the East of Flores, and whilst the exact arrival date in Sahul is disputed (The topic of a future article) it appears to be in the 47-60kya range. This makes an arrival date of Homo sapiens around 50kya in Flores quite reasonable. Additional evidence of a shift around 50kya is the increased prevalence of marine invertebrates at Liang Bua, which could be consistent with a shift in hominin prey base (26). Perhaps the closest thing to a smoking gun when it comes to human arrival is the discovery of two teeth in unit 4 (46-47kya) which were considerably larger than teeth associated with Homo floresiensis, and likely attributable to Homo sapiens. Thus, it can be quite speculated that Homo Sapiens arrived sometime between 50kya and 46kya. A human-caused extinction would fit the evidence of the Flores extinctions quite well. In unit 2 spanning 50-60kya is evidence of Homo floresiensis, Stegodon florensis, Leptoptilus robustus, and Trigonops sp. But no evidence of Homo sapiens. Then comes unit 3 a volcanic layer devoid of fossils, then unit 4 which has strong evidence of Homo sapiens but lacks Homo floresiensis and the megafauna species. The chronology is very consistent with this hypothesis, unlike the available evidence of a climatic shift. What’s more this hypothesis would be applicable to Sumba and explain the extinction of Stegodon sumbaensis, unfortunately the scant record in Sumba makes it difficult to shed any further light on the subject.
We can therefore propose human agency as the likeliest root cause of the megafauna extinctions in Flores approximately 50-46kya and presumably on Sumba as well around the same time. The relatively small temporal gap between unit 2 (60-50kya) and unit 4 (47-46kya) suggests that this extinction took place within the span of just a few thousand years, at least at Liang Bua. Unfortunately lacking additional fossil bearing locations from this period, we are forced to infer from a single site. Given that the extinction event only appears to apply to Stegodon florensis and its scavengers, overhunting would seem the most reasonable mechanism for extinction as there’s no evidence of invasive species or habitat change at this time. Homo floresiensis may have been hunted directly, but was more displaced by Homo sapiens through competition as seems to have been the case with Homo neanderthalensis and other Pleistocene hominids. These extinctions left Flores with the Komodo dragon as the sole surviving megafauna, however these losses did not mark the finale of the decline of the Floresian fauna. The Holocene would claim additional victims and introduce much of the wildlife we associate with the island today.
Figure 6. Timeline of units at Liang Bua and key events in Flores, green denoting units with megafauna present, yellow denoting units not bearing fossils and red lacking megafauna.
Terms of use: Own work
The Holocene Epilogue
The changes that occurred between the end of the Pleistocene and the modern day in Flores could easily fill an article by itself, however in this instalment a summary will have to suffice. In fact, whilst the Flores megafauna succumbed during the Pleistocene, it was during the Holocene that Flores saw the largest upheaval in its fauna. The difference in the vertebrate community between Liang Bua’s unit 7 (13-12kya) and unit 8a (12-5kya) - which marked the Pleistocene-Holocene transition - doubled when comparing to previous unit changes. The degree of change further doubled between units 8a and 8b (5-3kya) and the transition from 8b to 8c (3kya-present) also is higher than between any of the Pleistocene units (26). Given the relatively narrow time period of these shifts, it marks a radical departure from the ecology of Pleistocene Liang Bua. Several extinctions also take place during the Holocene including the disappearance of most of Flores’ large murids in the late Holocene including: Spelaeomys florensis, Papagomys theodorverhoeveni and Komodomys rintjanus (on Flores) (7, 8) and Milimonggamys juliae and Raksasamys tikusbesar on Sumba (28). Varanus hooijeri also appears to subsist into the Late Holocene at Liang Bua, though its remains are often difficult to distinguish from V. komodoensis placing slight uncertainty on this extinction time. Likely the Sumban equivalent goes extinct at a similar time though the lack of Sumban remains makes this difficult to ascertain.
What might be the causes of these extinctions? In this case the Holocene extinctions are almost indisputably a product of human agency. The primary mechanism in play appears to have been the introduction of novel species of which there are many, introduced species first show up in the early Holocene (Unit 8a) but really become plentiful in the middle and late Holocene (Units 8B and 8C) (26). During the Holocene various species of rodents were introduced including the brown rat (Rattus norvegicus), black rat (Rattus rattus), Polynesian rat (Rattus exulans), and ricefield rat (Rattus argentiventer) all of which likely competed with the endemic native rats and ultimately drove them to extinction (33). Flores also saw the influx of various species of medium-sized or large animals including: the Sunda sambar (Rusa timorensis), wild boar (Sus scrofa), Sunda porcupine (Hystrix javanica), Asian palm civet (Paradoxurus hermaphroditus), crab-eating macaque (Macaca fascicularis), dog (Canis familiaris), and more (26). In addition to these invasive species, an increase in human population size and changes in land use for agriculture probably contributed to placing pressure on the native Floresian species (7, 8). Today the fauna of Flores is almost unrecognizable, having lost almost all endemic species over 1kg in size and replaced them with species introduced from Southeast Asia and beyond. Only the Komodo Dragon clings on as a relic of a forgotten age.
Figure 7. The brown rat (Rattus norvegicus) is one of a number of introduced murids and was likely in direct competition with the large endemic rats on Flores.
Terms of use: This image is licensed under a Creative Commons Attribution 2.0 Generic. It is attributed to Peter O’Connor. The image is unedited and can be found here.
References
1. Brongersma, L. D.. (1958). On an extinct species of the genus Varanus (Reptilia, Sauria) from the island of Flores. Zoologische Mededelingen 7, 113-125.
2. Brown, P., Sutikna, T., Morwood, M. J., Soejono, R. P., Jatmiko, Saptomo, E. W., Due, R. A.. (2004). A new small-bodied hominin from the Late Pleistocene of Flores, Indonesia. Nature 431.
3. Brumm, A., Jensen, G. M., van den Bergh, G. D., Morwood, M. J., Kurniawan, I., Aziz, F., Storey, M.. (2010). Hominins on Flores, Indonesia, by one million years ago. Nature Letters 464, 748-752.
4. Brumm, A., van den Bergh, G. D., Storey, M., Kurniawan, I., Alloway, B. V., Setiawan, R., Setiyabudi, E., Grun, R., Moore, M. W., Yurnaldi, D., Puspaningrum, M. R., Wibowo, U. P.., Insani, H., Sutisna, I., Westgate, J. A., Pearce, N. J. G., Duval, M., Meijer, H. J. M., Aziz, F., Sutikna, T., van der Kaars, S., Flude, S., Morwood, M. J.. (2016). Age and context of the oldest known hominin fossils from Flores. Nature 524, 249-253.
5. Dennel, R., Louys, J., O’Regan, H. J., Wilkinson, D. M.. (2014). The origins and persistence of Homo floresiensis on Flores: biogeographical and ecological perspectives. Quarternary Science Reviews 96, 98-107.
6. Jessop, T., Ariefiandy, A., Azmi, M., Ciofi, C., Imansyah, J., Purwandana, D. (2021) Varanus komodoensis. The IUCN Red List of Threatened Species 2021: e.T22884A123633058.
7. Locatelli, E., Due, R. A., van den Bergh, G. D., Ostende, L. W. vd H.. (2012) Pleistocene survivors and Holocene extinctions: The giant rats from Liang Bua (Flores, Indonesia). Quaternary International 281, 47-57.
8. Locatelli, E., Due, R. A., Jatmiko, Ostende, L. W. vdH.. (2015). Middle-sized murids from Liang Bua (Flores, Indonesia): Insular endemics, human introductions and palaeoenvironment. Palaeobio Palaeoenv 95, 497-512.
9. Meijer, H. J. M., Due, R. A.. (2010a) A new species of giant s stork (Aves: Ciconiiformes) from the Pleistocene of Liang Bua, Flores (Indonesia). Zoological journal of the linnaen society 160, 707-724.
10. Meijer, H. J. M., van den Hoek Ostende, L. W., van den Bergh, G. D., de Vos, J.. (2010b) The fellowship of the hobbit: the fauna surrounding Homo floresiensis. Journal of Biogeography 37, 995-1006.
11. Meijer, H. J. M., Sutikna, T., Saptomo, E. W., Due, R. A., Wasisto, S., James, H. F., Morwood, M. J., Tocheri, M. W.. (2013). Late Pleistocene-Holocene non-passerine avifauna of Liang Bua (Flores, Indonesia). Journal of vertebrate palaeontology 33(4), 877-894.
12. Meijer, H. J. M., Kurniawan, I., Setiabudi, E., Brumm, A., Sutikna T., Setiawan, R., Van den Bergh, G. D. (2015) Avian remains from the Early/Middle Pleistocene of the So'a Basin, central Flores, Indonesia, and their palaeoenvironmental significance. Palaeogeography, Palaeoclimatology, Palaeoecology (440), 161-171.
13. Meijer, H. J. M., Due, R. A., Sutikna, T., Saptomo, W., Jatmiko, Wasisto, S., Tocheri, M. W., Mayr, G.. (2017) Late Pleistocene songbirds of Liang Bua (Flores, Indonesia); the first fossil passeroine fauna described from Wallacea. PeerJ 5 :e3676
14. Meijer, H. J. M., Louys, J., O’Connor, S.. (2019) First record of avian extinctions from the Late Pleistocene and Holocene of Timor Leste. Quaternary Science Reviews 203, 170-184.
15. Meijer, H. J. M., Sutikna, T., Saptomo, E. W., Tocheri, M. W.. (2022) More bones of Leptoptilos robustus from Flores reveal new insights into giant marabou stork paleobiology and biogeography. Royal Society Opem Science 9, 220435.
16. Musser, G. G., Boeadi.. (1980) A New Genus of Murid Rodent from the Komodo Islands in Nusatenggara, Indonesia. Journal of Mammalogy 61 (3), 395-419.
17. Musser, G. G.. (1981) The Giant Rats of Flores and Its Relatives East of Borneo and Bali. Bulletin of the American Museum of Natural History 169 (2), 71-175.
18. Ostende, L. W. vdH., van der Berch, G., Due, R. A.. (2006). First fossil insectivores from Flores. Hellenic Journal of Geosciences 41, 67-72.
19. Ostende, L. W. vdH., Zijlstra, J., Locatelli, E.. (2011) A giant shrew rat from Flores. Late Cenozoic Mammals: Fossil Record, Biostratigraphy, Paleoecology. International Colloquium in Honor of Prof. Old rich Fejfar: Program, Abstracts and an Excursion Guide. The Institute of Geology AS CV v.v.i., Prague.
20. Ouwendijk, E. M., Awe Due, R., Locatelli, E., Jatmiko, van den Hoek Ostende, L. (2014). Bat cave and hobbit hole, microbats of Liang Bua (Flores, Indonesia). Alcheringa: An Australasian Journal of Palaeontology 38(3), 422-433.
21. Rhodin, A. G. J., Thomson, S., Georgalis, G. L., Karl, H-V., Danilov, I. G.,Takahashi, A., de la Fuente, M. S., Bourque, J. R., Delfino, M., Bour, R., Iverson, J. B., Shaffer, H. B., van Dijk, P. P.. (2015). Turtles and Tortoises of the World During the Rise and Global Spread of Humanity: First Checklist and Review of Extinct Pleistocene and Holocene Chelonians. Conservation Biology of Freshwater Turtles and Tortoises Chelonian Research Monographs 5.
22. Scroxton, N., Gagan, M. K., Ayliffe, L. K., Hellstrom, J., Cheng, H., Edwards, R., Zhao, J., Hantoro, W. S., Rifai, H., Scott-Gagan, H., Cowley, J. A., Suwargadi, B. W.. (2013). Speleothem carbon isotopes in the tropics: a proxy for vegetation and what they reveal about the demise of Homo floresiensis. Amergican Geophysical Union Fall Meeting.
23. Scroxton, N., Gagan, M. K., Ayliffe, L. K., Zhao, J., Hantoro, W. S., Hellstrom, J., Cheng, H., Edwards, R., Zhao, J. X., Suwargadi, B. W., Scott-Gagan, H., Cowley, J. A., Rifai, H.. (2015). The Flores speleothem carbon isotope record: vegetation, volcanism and the demise of Homo Floresiensis. Amergican Geophysical Union Fall Meeting
24. Setiyabudi, E., Kurniawan, I., van den Bergh, G.. (2012) Fossils of Stegodon and Varanus komodoensis Sumba and Flores: A Pleistocene landbridge? Proceedings Pit Iagi Yogyakarta the 41st IAGI annual convention and Exhibition.
25. Sutikna, T., Tocheri, M. W., Morwood, M. J., Saptomo, E. W., Jatmiko, Due Awe, R., Wasisto, S., Westaway, K. E., Aubert, M., Li, B., Zhao, J-X., Storey, M., Alloway, B. V., Morley, M. W., Meijer, H. J. M., van den Bergh, G. D., Grun, R., Dosseto, A., Brumm, A., Jungers, W. L., Roberts, R. G. (2016a). Revisted stratigraphy and chronology for Homo floresiensis at Liang Bua in Indonesia. Faculty of Science, Medicine and Health – Papers: part A. 3378.
26. Sutikna, T. (2016b) New archaeological research at Liang Bua on the island of Flores: implications for the extinction of Homo floresiensis and the arrival of Homo sapiens in eastern Indonesia. PhD Thesis University of Wollongong.
27. Sutikna, T., Tocheri, M. W., Faith, J. T., Jatmiko, Due Awe, R., Meijer, H. J. M., Saptomo, E. W., Roberts, R. G. (2018) The spatio-temporal distribution of archaeological and faunal finds at Liang Bua (Flores, Indonesia) in light of the revised chronology for Homo floresiensis
28. Turvey, S. T., Crees, J. J., Hansford, J., Jeffree, T. E., Crumpton, N., Kurniawan, I., Setiyabudi, E., Guillerne, T., Paranggarimu, U., Dosseto, A., van den Bergh, G. D.. (2017) Quarternary vertebrate faunas from Sumba, Indoniesa: implications for Wallacean biogeography and evolution. Proceedings of the Royal Society of Biological Sciences 284: 20171278.
29. Van den Bergh, G. D., Kaifu, Y., Kurniawan, I., Kono, R. T., Brumm, A., Setiyabudi, E., Aziz, F., Morwood, M. J.. (2016). Homo floresiensis–like fossils from the early middle Pleistocene of Flores. Nature letters 534, 245-248.
30. Van der Geer, A. A. E., Lyras, G. A., de Vos, J., Dermitzakis, M.. (2010). Evolution of island mammals: Adaptation and Extinction of Placental Mammals on Islands. Chapter 13: Flores. Wiley-Blackwell, Chichester. 190-205.
31. Van der Geer, A. A. E., Van den Bergh, G. D., Lyras, G. A., Prasetyo, U. W., Awe Due, R., Setiyabudi, E., Drinia, H. (2016) The effect of area and isolation on insular dwarf proboscideans. Journal of Biogeography 43, 1656-1666.
32. Westaway, K. E., Morwood, M. J., Sutikna, T., Moore, M. W., Rokus, A. D., Van den Bergh, G. D., Roberts, R. G., Saptomo, E. W. (2009). Homo floresiensis and the late Pleistocene environments of eastern Indonesia: defining the nature of the relationship. Quarternary Science Reviews 28, 2897-2912.
33. Veatch, E. G., Tocheri, M. W., Sutikna, T., McGrath, K., Saptomo, E. W., Jatmiko, Helgen, K. M. (2019). Temporal shifts in the distribution of murine rodent body size classes at Liang Bua (Flores, Indonesia) reveal new insights into the paleoecology of Homo floresiensis and associated fauna. Journal of Human Evolution 130, 45-60.
34. Powley, M. J., Sutisna, I., Mikac, K. M., Wibowo, U. P., van den Bergh, G. D. (2021). The Stegodon Bonebed of the Middle Pleistocene Archaeological Site Mata Menge (Flores, Indonesia): Taphonomic Agents in Site Formation. Quarternary 4 (31), 1-20.