Changing the Guard: Extinction and Migration in Ice Age America
Introduction
At the end of the Pleistocene, North America saw the extinction of about 70% of its megafauna guild (37); a catastrophic event, the cause of which is fiercely debated today. The magnitude of this loss may however be a conservative figure, a suspicion that arises when perusing the list of ‘surviving’ mammalian megafauna, because many also are present in Eurasia. These species are moose (Alces alces), wapiti (Cervus canadensis), caribou (Rangifer tarandus), muskox (Ovibos moschatos), brown bear (Ursus arctos), polar bear (Ursus maritimus), and grey wolf (Canis lupus). Whilst these taxa could have survived the terminal Pleistocene in North America – It is conceivable that they instead arrived during or after the extinctions took place. Though this difference may seem trivial, it would affect the dynamics of studies and underplay the severity of the faunal loss of North America.
To investigate whether these species constitute recent arrivals to North America, it is key to look at two sources of information: palaeontology & phylogeography. Fossil records can supply us with concrete evidence of when and where a taxon was present, if properly dated these data points can be quite exact. This source of evidence is not without caveats though, remains may be misclassified or erroneously dated. Furthermore, the absence of a species in the record is not necessarily proof of a late arrival but may occur due to poor regional fossilisation, low population densities or other biases. It can therefore be helpful to supplement with phylogeography – the study of the genetic interrelationship between populations in different places. By understanding how closely related populations in North America are, it is possible to estimate the most recent common ancestor, which may indicate the colonisation time. The genetic proximity to populations in Eurasia and to extinct individuals may further shed light on when and how a species arrived in North America.
One further guide to understanding when species arrived in North America is the geography of the continent during the Pleistocene. North America was connected to Asia via the Beringian land bridge, allowing the movement of some species across the two continents during glacial periods when sea levels were low – this includes during the Late Pleistocene until about 11kya when the modern iteration of the ‘Bering strait’ arose (16). Another key feature was the Cordilleran-Laurentide Ice Sheet (CLIS)– A glacier which blocked the movement of species between ‘Beringia’ (Modern day Siberia, Alaska and Yukon) and the rest of North America during periods of glaciation. At various times during the Pleistocene (5, 30) an ice-free corridor in the CLIS is known to have connected the two regions at various times, most recently opening up 13-14kya (5) but also during various points of the Late Pleistocene (30).
In this article we shall dispense with the origins of the tundra-fauna (Perhaps an article for another day) and simply focus on the four Eurasian species which can be found in the North America mid-continent (the area south of the CLIS): wapiti, moose, brown bear & grey wolf.
Wapiti
Fig 1. A male Wapiti/Elk (Cervus canadensis)
Terms of use: No Rights Reserved
Cervus canadensis, commonly known as the elk or wapiti, (As we shall call it here to avoid conflation with moose, which is also frequently called ‘elk’) is prolific in the American landscape, but it has been posited it is a newcomer to the continent.
In North America the first reported finds of Cervus canadensis (or synonymously as Cervus elaphus) emerge during the Late Pleistocene (Rancholabrean, 250-11kya) (3). The earliest of these date to Marine isotopic stage (MIS) 5 (71-130kya), and additional fossils are reported from MIS 2-4 (71-14kya). These fossils are fragmentary and thus difficult to confidently assign to C. canadensis. The issue of taxonomic conflation is especially egregious in Cervids where post-cranial remains are very similar (3), indeed, several putative C. canadensis remains have so far been: reassigned to Cervalces scotti (Commonly dubbed the Elk-Moose), reclassified to younger strata, or – quite comically –found not to belong to cervids at all (25). The first unambiguous evidence of wapiti in the New World hails from Eastern Beringia around 15kya (25).
It is worth pausing the exploration for a moment and considering that wapiti populations subsisted in Beringia , considerably north of their present range. This does not appear a brief aberration either, with continuous remains in Alaska until about 5kya (25) and in Siberia until just 500 years ago! (25).
Definite fossils of Cervus canadensis follow in the midcontinent shortly after, the earliest from North Ohio around 12kya (29). An abundance of additional material succeeds this specimen throughout Canada and the United States with an apparent gradient of younger first occurrences when moving southwards, suggesting the gradual colonisation of the continent from the north (3).
To supplement fossil data, phylogeographic analyses can be used to determine the interrelations of sub-populations, estimate divergence times, and evaluate biogeographical scenarios. Unfortunately, no such studies have been conducted with focus on the North American populations of C. canadensis, though some have investigated the global relationships between subpopulations of C. elaphus and C. canadensis (15, 21, 22). Divergence times were not estimated, but the investigations did suggest that modern American wapiti form a single close-related clade, and thus likely originated from a single recent migration event (15, 21, 22).
Based on the fossil and genetic evidence available, it seems that wapiti colonised North America only at the very end of the Pleistocene – Beringia around 15kya and the mid-continent shortly thereafter. Whilst it is possible that some earlier fossils do indeed belong to C. canadensis, there is little evidence to believe this to be true. Furthermore, genetic evidence supports that all modern wapiti are descended from a single colonisation, thus even had an early population reached North America it does not appear to have given rise to the modern N. American wapiti.
Moose
Fig 2. Bull Moose/Elk (Alces alces)
Terms of use: This image is licensed under an Attribution 2.0 Generic. It is attributed to The Government of Alberta and is unedited
Another cervid has also been suggested as immigrating to America during the Pleistocene terminus, Alces alces - also commonly known as the moose (or elk). As with wapiti, several remains are reported from the Pleistocene, but these are fragmentary and/or indirectly dated (10, 19) and must be treated with scepticism when attempting to construct a timeline. It is around 15kya - contemporarily with the wapiti - that the first definite fossil of Alces alces appear in Alaska.
Unfortunately, it is not clear when the movement into the mid-continent began. whilst Beringia is fossiliferous during the Late Pleistocene and Early Holocene, moose fossils elsewhere in this period are few and far between, thus tracking their dispersal is difficult (3, 26). It appears the species arrived sometime around the early Holocene where a few early fossils are known from (3).
The genetic evidence complements the fossil record quite well. A 2002 study by Hundertmark et al, found that all North American moose are derived from most recent common ancestor (MRCA), likely dating to sometime to sometime after the Last Glacial Maximum (26-20kya). This is further corroborated by DeCesare et al 2020, who also retrieved a recent common origin for the modern North American populations.
One point of evidence that may have put the arrival date into doubt is the relatively large genetic distance between the American populations of A.alces and those inhabiting modern day Siberia (Western Beringia). However, sequencing of DNA from Pleistocene specimens reveal a much closer relationship, indicating that the original Siberian population that colonised America has since gone extinct, likely displaced by the population inhabiting the region today (6).
One final consideration is the disproportionately high vulnerability of A. alces to winter ticks (Dermacentor albipictus) when compared to other cervids. This may be a result of lack of evolutionary adaptations against the American parasite, due to the recency of moose colonisation. Although, why Wapiti don’t face similar pressures is unclear. The picture of moose colonisation is relatively clear, it appears the species colonised Beringia concurrently with wapiti around 15kya and then subsequently migrated South, though the exact timing of this is unclear.
Fig 3. Timeline of colonisation events mentioned in text for the wapiti (Cervus canadensis) & moose (Alces alces)
Terms of use: Own Work
Brown Bears
Fig 4. A North American Brown Bear (Ursus arctos horribilis)
Terms of use: No Rights Reserved
Traditionally it has been thought that Brown bears (Ursus arctos) also were a recent migrant to the American mid-continent (Though not Beringia), coinciding with the opening of the ice-free corridor at the end of the Pleistocene. Research over the last two decades has put this hypothesis into question.
Looking firstly at the fossil evidence, only a few pre-Holocene sites were thought to contain brown bear fossils, and these were all putative, fragmentary and indirectly dated (8). This changed with the discovery of a brown bear specimen from Alberta directly dated to about 26kya, confirming the presence of the species in the mid-continent earlier than initially postulated (23). This site was in the ice-free corridor and so doesn’t demonstrate bears south of the CLIS, but heavily implies it. This is in many ways unsurprising, because although brown bears are absent from the mid-continental fossil record at this time, they are well documented from Eastern Beringia over the last 100kya (30). Only a noticeable absence in the Beringia is recorded, around 34-20kya (2).
Early genetic studies suggest that the mid-continent of North America was colonised by a single lineage of brown bears, known in the literature as ‘clade 4’. Other distinct clades are known from North America. Clade 2a contains brown bears in the ABC islands (Admiralty, Baranof & Chichagof) and 2b represents Polar bears (34). Clade 3a inhabits East Beringia & Eurasia (17).
It has been suggested that all these clades were present in a single diverse Beringian population prior to 35kya, and that clades 2a & 4 were simply the founders of the ABC and mid-continental populations respectively, the latter suggested to occur at the very end of the Pleistocene (17) The sequencing of the Alberta specimen places it in clade 4 (23) which pushes back the colonisation date significantly.
Recent studies seem to suggest multiple separate colonisation events to North America, though only one to the mid-continent (2, 30). Clade 3a appears derived from a refugia in central Asia or the Carpathians around 45kya and constitutes a separate colonisation event to Beringia around 20kya (1). A study focused on the highly localized clade 2a, suggested that it may be a population descended from admixture between polar bears and primarily male brown bears, which explains the close relationship to modern polar bears (4). The clade is thought to have originated approximately 17kya (30).
Perhaps most enlightening is a study by Salis et al 2021, looking at the precise patterns of brown bear haplotype occurrences in Beringia and estimating divergence times using ancient genomics and radiocarbon dating. Herein it was estimated that members of clade 4 shared a MRCA approximately 92kya. When they dispersed south to the mid-continent is less clear, they disappear from Beringian fossil record around 35kya, which places a tentative last possible date for the migration. Salis et al 2021 suggests the MIS 5 warm period (130-71kya), when other mammalian species such as the red fox (Vulpes vulpes), Bison priscus, and the American Lion (Panthera atrox) are thought to have immigrated (30).
The picture of brown bear migration into North America is far more complex than those of the moose or wapiti. Nevertheless, recent studies have painted a compelling picture of a multi-wave colonisation of Beringia with a single founding population reaching the mid-continent between MIS 5 and 3.
Fig 5. Timeline of colonization events mentioned in text for the brown bear (Ursus arctos)
Terms of use: Own Work
Grey Wolves
Fig 6. A pack of Beringian wolves (Canis lupus) hunting woolly mammoth (Mammuthus primigenius) in Pleistocene Yukon
Terms of use: Artwork by Hodari Nundu and used with the permission of the artist
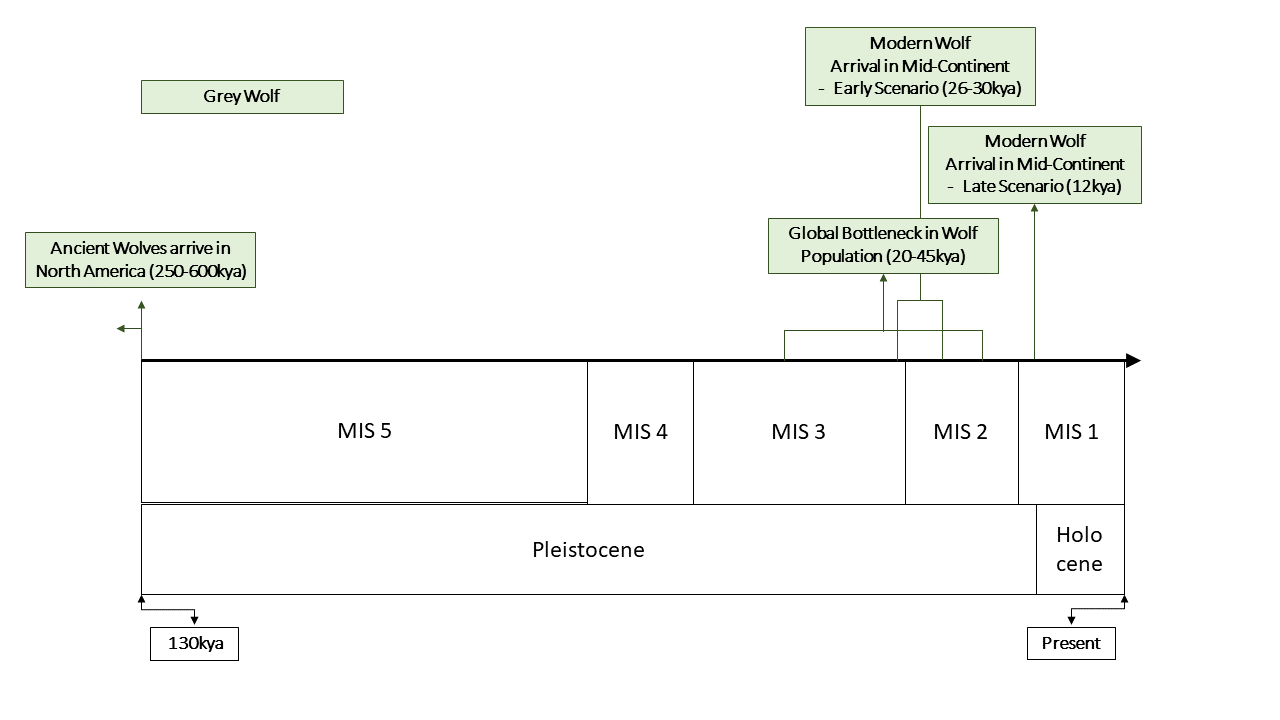
The final species to be investigated is the grey wolf (Canis lupus). Grey wolves are known from North America since the Middle Pleistocene (Irvingtonian, 600-250kya) and there is no paucity in their fossil record (32). Despite this, considerable evidence suggests that the current population may be derived from a recent arrival, perhaps at the end of the Pleistocene.
The first line of evidence that could suggest a recent colonisation are the morphological differences between Pleistocene and modern grey wolf populations. Several Late Pleistocene specimens exhibit traits associated with megafauna hunting such as enlarged carnassials and larger skulls (18). This morphotype, commonly called the ‘Beringian wolf’ has been discovered in North America on both sides of the CLIS (24). However, populations of grey wolf similar in skull morphology to modern day animals are also known from Late Pleistocene continental North America (18). The relationship of these wolves with the modern populations is yet untested. Remains of the Beringian wolf are not known to have co-existed with the smaller contemporary morphotypes (8, 18, 24).
Further elucidating the distinction between modern grey wolves and their Pleistocene counterparts is limb morphology. Simply put, modern North American wolves are significantly more cursorial than specimens known from the Pleistocene (33). This trait, however, does not appear until well into the Holocene epoch, nor is it entirely ubiquitous within modern American wolves as both the Mexican wolf (Canis lupus baileyi) and the extirpated Great Plains wolf (Canis lupus nubilus) are short-legged (33). Limited stock can then be placed in morphology to track the movement of modern wolves into America, instead phylogenetics must be relied on.
A 2010 study found that modern North American Grey wolves likely originated from three distinct waves from Eurasia. A timeframe is not provided, though it is probably best explained as various interglacials. The Mexican wolf is suggested to originate from an initial dispersal due to its basal position (35), Great Plains wolves from a second wave and Mackenzie valley wolves (Canis lupus occidentalis) from a recent colonisation – Possibly at the onset of the interglacial (35).
More recent genetic analyses of dogs and wolves suggest that all modern wolves underwent a recent genetic bottleneck during the Late Pleistocene (7, 11, 14, 20, 31). This bottleneck is variously dated at 20-45kya (11, 14, 20), if so, several colonisation events are unlikely given the narrow corridor of dispersal.
An extensive study was carried out by Loog et al in 2019 with extensive sampling of up to 50ky old aDNA. The study pinned down the location of the bottleneck to Beringia and estimated it as occurring 24kya. If true, modern North American wolves must have been unable to reach the mid-continent until after the emergence of the ice-free corridor (20, 27). An alternative scenario is presented by Koblmuller et al 2016 which dated the split between North American and Eurasian populations sometime before 30kya, suggesting a colonisation event prior to the LGM (7, 14).
The sequencing of two Pleistocene wolves from the Late Pleistocene of Western Beringia (14.1kya & 16.8kya respectively), suggested that they may cluster with modern North American wolves, rather than Eurasian (28), this genetic proximity could indicate a colonisation event close in time to the two specimens.
Resolving the interrelationships of wolves and quantifying when present populations diverged is exceedingly difficult due to interbreeding between grey wolves and other canids - particularly golden jackals (Canis aureus) in Eurasia, and coyotes (Canis latrans) in America (28).
Throwing further complication into the equation is the Mexican wolf, which does not cluster with other North American wolves in either the study by Koblmuller et al 2016 or Loog et al 2019. A more recent 2021 paper by Wilson & Rutledge found that Mexican wolves in fact cluster closely to the extinct Beringian wolves, suggesting a degree of interbreeding during the Late Pleistocene (36). Caution should certainly be taken in interpreting these results, because the bottleneck is so recent few genetic differences have accumulated and so these phylogenetic analyses are sensitive to not just interbreeding but also high rates of evolution driven by natural selection or drift (14).
Nevertheless, the Mexican wolf adds a huge question mark to the narrative of a single migration at the end of the Pleistocene, and future studies will need to clear up the confusion surrounding this sub-species before a clear picture can emerge. If the Mexican wolf constitutes a separate colonisation or a hybridisation event with incumbent populations, then it becomes fairly evident that other American wolves are descended from a single migration, either before the LGM or at the very end of the Pleistocene.
Fig 7. Timeline of colonization events mentioned in text for the Grey Wolf (Canis lupus)
Terms of use: Own Work
Conclusion
Amongst the mid-continent species with Eurasian distributions only the modern brown bear can confidently be said to have survived the late Pleistocene extinction event in North America, with its colonisation likely extending back to an earlier point in the Late Pleistocene. There is very little evidence supporting the presence of either moose or wapiti in North America prior to their arrival in Beringia 15kya, and the two species appear to arrive in the mid-continent concurrently with the extinction of the megafauna. For this reason, the two cervid species should be carefully considered and discussed in studies related to the terminal Pleistocene extinction. Finally, the grey wolf presents the most puzzling picture, it can be confidently traced in the North American fossil record, however modern wolves appear to be descended from a single wave which quite possibly occurred at the end of the Pleistocene as with wapiti and moose. The wave could however have occurred earlier and the controversy surrounding the Mexican wolf could suggest interbreeding with extinct populations or an earlier independent wave. These nuances are important to resolve as they determine whether the grey wolf was a survivor or a victim of the extinction.
References
1. Anijalg, P., Ho, S. Y. W., Davison, J., Keis, M., Tammeleht, E., Bobowik, K., Tumanov, I. L., Saveljev, A. P., Lyapunova, E. A., Vorobiev, A. A., Markov, N. L,. Kryukov, A. P., Kojola, I., Swenson, J. E., Hagen, S. B., Eiken, H. G., Paule, L., Saama, U.. (2019). Large-scale migrations of brown bears in Eurasia and to North America during the Late Pleistocene. Journal of Biogeography 45, 398-405.
2. Barnes, I., Matheus, P., Shapiro, B., Jensen, D., Cooper, A.. (2002) Dynamics of Pleistocene Population Extinctions in Beringian Bronw Bears. Science 295, 2267-2270.
3. Burns, J. A.. (2010) Mammalian faunal dynamics in Late Pleistocene Alberta, Canada. Quarternary International 217, 37-42.
4. Cahill, J. A., Green, R. E., Fulton, T. L., Stiller, M., Jay, F., Ovsyaniko, N., Salamzade, R., St. John, J., Stirling, I., Slatkin, M., Shapiro, B.. (2013) Genomic Evidence for Island Population Conversion Resolves Conflicting Theories of Polar Bear Evolution. PLOS Genetics 9(3) e1003345.
5. Clark, J., Carlson, A. E., Reyes, A. V., Rood, D. H.. (2022). The age of the opening of the Ice-Free Corridor and implications for the peopling of the Americas. PNAS 119(14) e2118558119
6. DeCesare, N. J., Weckworth, B. V., Pilgrim, K. L., Walker, A. B. D., Bergman, E. J., Colson, K. E., Corrigan, R., Harris, R. B., Hebblewhite, M., Jesmer, B. R., Newby, J. R., Smith, J. R., Tether, R. B., Thomas, T. P., Schwartz, M. K.. (2020). Phylogeography of moose in western North America. Journal of Mammology 101(1), 10-23.
7. Fan, Z., Silva, P., Gronau, I., Wang, S., Armero, A. S., Schweizer, R. M., Ramirez, O., Pollinger, J., Galavemi, M., Del-Vecchyo, D. O., Du, L., Zhang, W., Zhang, Z., Xing, J., Vila, C., Marques-Bonet, T., Godinho, R., Yue, B., Wayne, R. K.. (2016) Worldwide patterns of genomic variation and admixture in gray wolves. Genome research 26, 163-173.
8. Feranec, R. S., Hadly, E. A., Blois, J. L., Barnosky, A. D., Paytan, A.. (2007) Radiocarbon dates from the Pleistocene fossil deposits of Samwel Cave, Shasta county, California, USA. Radiocarbon 49(1), 117-121.
9. Fox-Dobbs, K., Leonard, J. A., Koch, P. L.. (2008) Pleistocene Megafauna from eastern Beringia: Paleoecological and palaeonvironmental interpretations of stable carbon and nitrogen isotope and radiocarbon records. Paleogeography, palaeoclimatology, palaeoecology 261, 30-46.
10. Fisher, D. C., Shoshani, J., Zawiskie, J. M., Thurlow, S. J., Shoshani, S. L., Benninghoff, W. S., Zoch, F. H.. (1989) The Shelton mastodon site: Multidisciplinary study of a Late Pleistocene (Twocreekan) locality in southeastern Michigan. Contributions from the Museum of Paleontology the University of Michigan 27(14), 393-436.
11. Freedman, A. H., Gronau, I., Schweizer, R. M., Vecchyo, D. O-D., Han, E., Silva, P. M., Galaverni, M., Fan, Z., Marx, P., Lorente-Galdos, B., Beala, H., Ramirez, O., Hormozdiari, F., Alkan, C., Vila, C., Squire, K., Geffen, E., Kusak, J., Boyko, A. R., Parker, H. G., Lee, C., Tadigotla, V., Siepel, A., Bustamente, C. D., Harkens, T. T., Nelson, S. F., Ostrander, E. A., Marques-Bonet, T., Wayne, R. K., Novembre, J.. (2014) Genome Sequencing Highlights the Dynamic Early History of Dogs. PLOS Genetics 10(1) e1004016
12. Guthrie, R. D.. (2006) New carbon dates link climatic change with human colonization and Pleistocene extinctions. Nature Letters 441, 207-209.
13. Hundertmark, K. J., Shields, G. F., Udina, I. G., Bowyer, R. T., Danikin, A. A., Schwartz, C. C.. (2002) Mitochondrial Phylogeography of Moose (Alces alces) Late Pleistocene Divergence and Population Expansion. Molecular Phylogenetics and Evolution 22(3), 375-387.
14. Koblmuller, S., Vila, C., Lorente-Galdos, B., Dabad, M., Ramirez, O., Marques-Bonet, T., Wayne, R. K., Leonard, J. A.. (2016) Whole mitochondrial genomes illuminate ancient intercontinental dispersals of grey wolves (Canis lupus). Journal of Biogeography 43, 1728-1738.
15. Kuznetsova, M. V., Danilkin, A. A., Kholodova, M. V.. (2011) Phylogeography of Red Deer (Cervus elaphus) Analysis of mtDNA Cytochrome b Polymorphism. Biology Bulletin 39 (4), 323-330.
16. Jakobsson, M., Pearce, C., Cronin, T. M., Backman, J., Anderson, L. G., Barrientos, N., Bjork, G., Coxall, H., de Boer, A., Mayer, L. A., Morth, C-M., Nilsson, J., Rattray, J. E., Stranne, C., Semilietov, I., O’Regan, M.. (2017) Post-glacial flooding of the Beringia Land Bridge dated to 11,000 cal yrs BP based on new geophysical and sediment records. Climate of the Past Discussions.
17. Leonard, J. A., Wayne, R. K., Cooper, A.. (2000) Population genetics of Ice Age Brown bears. PNAS 97(4), 1651-1654.
18. Leonard, J. A., Vila, C., Fox-Dobbs, K., Koch, P. L., Wayne, R. K., van Valkenburg, B.. (2007) Megafaunal Extinctions and the Disappearance of a Specialized Wolf Ecomorph. Current Biology 17, 1146-1150.
19. Long, C. A.. (1971) Significance of the Late Pleistocene fauna from the Little Box Elder Cave, Wyoming, to studies of zoogeography of recent mammals. The Great Basin Naturalist 31(2), 93-105.
20. Loog, L., Thalmann, O., Sinding, M-H. S., Schuenemann, V. J., Perri, A., Germonpre, M., Bocherens, H., Witt, K. E., Castruita, J. A. S., Velasco, M. S., Lundstrøm, I. K. C., Wales, N., Sonet, G., Frantz, L., Schroeder, H., Budd, J., Jimenez, E-L., Fedorov, S., Gasparyan, B., Kandel, A. W., Laznickova-Galetova, M., Napierala, H., Uerpmann, H-P., Nikolskiy, P. A., Pavlova, E. Y., Pitulko, V. V., Herzig, K-H., Malhi, R. S., Willerslev, E., Hansen, A. J., Dobney, K., Gilbert, M. T. P., Krause, J., Larson, G., Erisson, A., Manica, A.. (2019) Ancient DNA suggests modern wolves trace their origin to a Late Pleistocene expansion from Beringia. Molecular Ecology 29, 1596-1610.
21. Ludt, C. J., Schroeder, W., Rottmann, O., Kuehn, R.. (2004) Mitochondrial DNA phylogeography of red deer (Cervus elaphus). Molecular Phylogenetics and Evolution 31, 1064-1083.
22. Mahmut, H., Masuda, R., Onuma, M., Takahashi, M., Nagata, J., Suzuki, M., Ohtaishi, N.. (2002) Molecular Phylogeography of the Red Deer (Cervus elaphus) populations in Xinjiang of China: Comparison with other Asian, European, and North American Populations. Zoological Science 19, 485-495.
23. Matheus, P., Burns, J., Weinstock, J., Hofreiter, M.. (2004) Pleistocene Brown Bears in the Mid-Continent of North America. Science 306, 1150-1151.
24. Meachen, J. A., Brannick, A. L., Fry, T. J.. (2016) Extinct Beringian wolf morphotypes found in the continental U.S. has implications for wolf migration and evolution. Ecology and Evolution 6(10), 3430-3438.
25. Meiri, M., Lister, A. M., Collins, M. J., Tuross, N., Goebel, T., Blockley, S., Zazula, G. D., van Doom, N., Guthrie, R. D., Boeskorov, G. G., Baryshnikov, G. F., Sher, A., Barnes, I.. (2014) Faunal record identifies Bering isthmus conditions as constraint to end-Pleistocene migration to the New World. Proceedings of the Royal Society of Biological Sciences 281, 20132167.
26. Meiri, M., Lister, A., Kosintsev, P., Zazula, G., Barnes, I.. (2020) Population dynamics and range shifts of moose (Alces alces) during the Late Quarternary. Journal of Biogeography 47, 2223-2234.
27. Pedersen, M. W., Ruter, A., Schweger, C., Friebe, H., Staff, R. A., Kjeldsen, K. K., Mendoza, M. L. Z., Beaudoin, A. B., Zutter, C., Larsen, N. K., Potter, B. A., Nielsen, R., Rainville, R. A., Orlando, L., Meltzer, D. J., Kjær, K. H., Willerslev, E.. (2016) Postglacial viability and colonization in North America’s ice-free corridor. Nature 537, 45-51.
28. Ramos-Madrigal, J., Sinding, M-H. S., Carøe, C., Mak, S. S. T., Niemann, J., Castruita, J. A. S., Fedorov, S., Kandyba, A., Germonpre, M., Bocherens, H., Feuerborn, T. R., Pitulko, V. V., Pavlova, E. Y., Nikolsky, P. A.. (2021). Genomes of Pleistocene Siberian Wolves Uncover Multiple Extinct Wolf Lineages. Current Biology 31, 198-206.
29. Redmond, B. G.. (2021) New record of Terminal Pleistocene Elk/Wapiti (Cervus canadensis) from Ohio, USA. Ohio Journal of Science 121(2), 2-14.
30. Salis, A. T., Bray, S. C. E., Lee, M. S. Y., Heiniger, H., Barnett, R., Burns, J. A., Doronichev, V., Fedje, D., Golovanova, L., Harington, C. R., Hockett, B., Kosintsev, P., Lai, X., Mackie, Q., Vasiliev, S., Weinstock, J., Yamaguchi, N., Meachen, J. A., Cooper, A., Mitchell, K. J.. (2021) Lions and brown bears colonized North America in multiple synchronous waves of dispersal across the Bering Land Bridge. Molecular Ecology, 1-15
31. Skoglund, P., Ersmark, E., Palkopoulou, E., Dalen, L.. (2015) Ancient Wolf Genome Reveals an Early Divergence of Domestic Dog Ancestors and Admixture into High-Latitude Breeds. Current Biology 25, 1515-1519.
32. Tedford, R. H., Wang, X., Taylor, B. E.. (2009) Phylogenetic Systematics of the North American fossil Caninae (Carnivora: Canidae). Bulletin of the American Museum of Natural History 325, 1-218.
33. Tomiya, S., Meachen, J. A.. (2018) Postcranial diversity and recent ecomorphic impoverishment of North American gray wolves. Biology Letters 14, 20170613.
34. Waits, L. P., Talbot, S. L., Ward, R. H., Shields, G. F.. (1998) Mitochondrial DNA Phylogeography of the North American Brown Bear and Implications for Conservation. Conservation Biology 12(2), 409-417.
35. Weckworth, B. V., Talbot, S. L., Cook, J. A.. (2010) Phylogeography of wolves (Canis lupus) in the Pacific Northwest. Journal of Mammalogy 91(2), 363-375.
36. Wilson, P. J., Rutledge, L. Y.. (2021) Considering Pleistocene North American wolves and coyotes in the eastern Canis origin story. Ecology and Evolution 11, 9137-9147.
37. Broughton, J. M., Weitzel, E. M.. (2018). Population reconstructions for humans and megafauna suggest mixed causes for North American Pleistocene extinctions. Nature communications 9, 5441